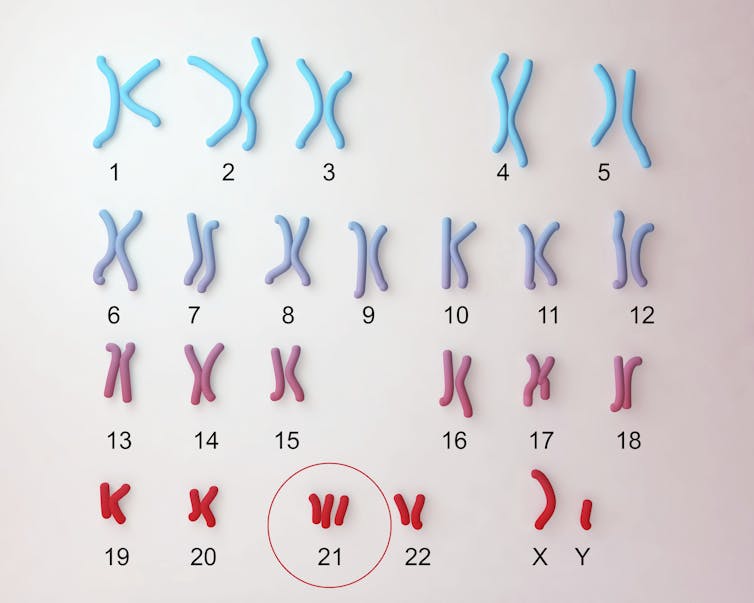
People with Down syndrome, or trisomy 21, a genetic condition caused by an extra copy of human chromosome 21, experienced a remarkable increase in life expectancy during the 20th century. In the early 1900s, less than 20% of newborns with Down syndrome survived past age 5. In the U.S. today, more than 90% of babies with this condition live past age 10 and have a life expectancy of nearly 60 years. These increases were likely fueled by greater inclusion in general society, the discontinuation of institutionalization in psychiatric facilities and better medical care.
Despite these advances, people with trisomy 21 experience an increased risk of many co-occurring conditions, such as congenital heart defects, autoimmune conditions, autism spectrum disorders and Alzheimer’s disease. On the other hand, people with Down syndrome tend to have lower levels of hypertension and certain types of cancers.
Kateryna Kon/Science Photo Library via Getty Images
Understanding how an extra chromosome 21 causes these risks and resiliencies could advance collective understanding of major medical conditions that also affect the general population. For example, the increased risk of Alzheimer’s disease among adults with Down syndrome can be explained in part by the presence of a gene on chromosome 21 that leads to excess production of the beta-amyloid proteins and plaques characteristic of Alzheimer’s.
In our newly published research, my research team and I found that genes involved in controlling the immune system are critical to the development of multiple hallmarks of Down syndrome. Our findings contribute to a growing body of research on the immune system’s important role in the appearance and severity of some of the negative health effects of trisomy 21, supporting the idea that restoring immune balance could help improve the quality of life of people with the condition.
When too much of a good thing is bad
The genes we identified, which encode what are called interferon receptors, are an important part of the immune system’s antiviral defense. These genes enable our cells to recognize a set of proteins called interferons, which virus-infected cells produce to alert the yet uninfected cells around them about the presence of a virus during an infection.
While interferons do trigger a beneficial immune response against viral infections, chronic interferon hyperactivity could have detrimental effects. Too much interferon signaling is known to be harmful in medical conditions such as systemic lupus erythematosus, a group of genetic disorders known as interferonopathies and severe COVID-19.
Nevit Dilmen/Wikimedia Commons, CC BY-SA
Notably, four of the six human interferon receptor genes are located on chromosome 21. Most people have only two copies of each chromosome and so would have only two copies of these genes. Because people with Down syndrome have three copies of chromosome 21, they also have three copies of the interferon receptor genes on it. This contributes to the overproduction of interferon receptors seen in those with Down syndrome.
Our team wanted to know whether this extra copy of interferon receptor genes, compared with the roughly 200 other genes located on chromosome 21, contribute to features of Down syndrome. To do this, we used a mouse model of Down syndrome. In this mouse model, a large region of its genome that is equivalent to a large portion of human chromosome 21 is triplicated to reproduce many features of Down syndrome.
Using CRISPR gene editing technology, we reduced the number of interferon receptor genes from three to the typical two, leaving all other triplicated genes intact. We found that correcting the number of interferon receptor genes significantly reduced abnormal gene expression patterns across multiple tissue types, both during embryonic development and in adult mice. These mice also had more regulated immune responses, normal heart development, reduced developmental delays, improved performance on memory and learning tasks and even a more typical skull and facial morphology.
Overall, our findings suggest that the tripling of interferon receptor genes may cause a number of key traits of Down syndrome.
Therapeutic implications and future directions
Our research indicates that many, though not all, aspects of Down syndrome may be associated with hyperactivity of the immune system’s interferon response. It also supports the possibility of using drugs that attenuate this response to treat some of the negative health effects of trisomy 21.
Our team is currently leading two clinical trials to test the safety and efficacy of one such drug, tofacitinib (Xeljanz). This drug belongs to a class of drugs known as JAK inhibitors used to treat autoinflammatory conditions. One trial focuses on autoimmune skin conditions more common in Down syndrome. The second trial focuses on Down syndrome regression disorder, or DSRD, a rare but devastating neurological condition that can result in loss of speech, sleep disruptions, difficulty moving and hallucinations. There is evidence that suggests that a subset of DSRD cases may be caused by immune dysregulation affecting the brain.

Flashpop/DigitalVision via Getty Images
Our study findings also support further investigation into the effects of interferon hyperactivity on fetal development more generally. Two of the key traits of Down syndrome that we found were affected by the tripling of interferon receptors – congenital heart disease and skull and facial shape – develop in utero.
Though our research shows promise on the potential of JAK inhibitors and other drugs that modulate the immune system to improve health outcomes in Down syndrome, more research in people is needed to determine their safety and efficacy.



